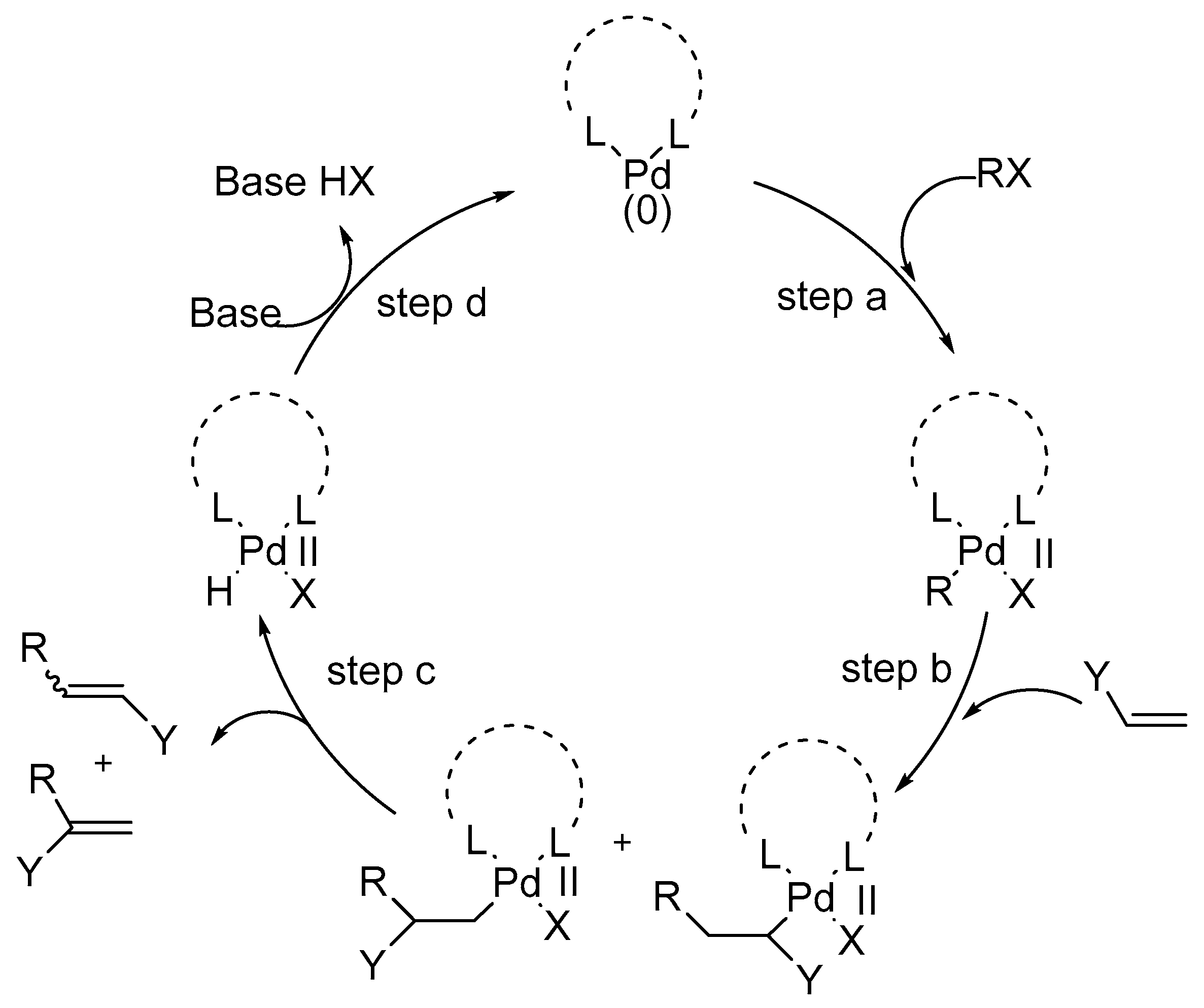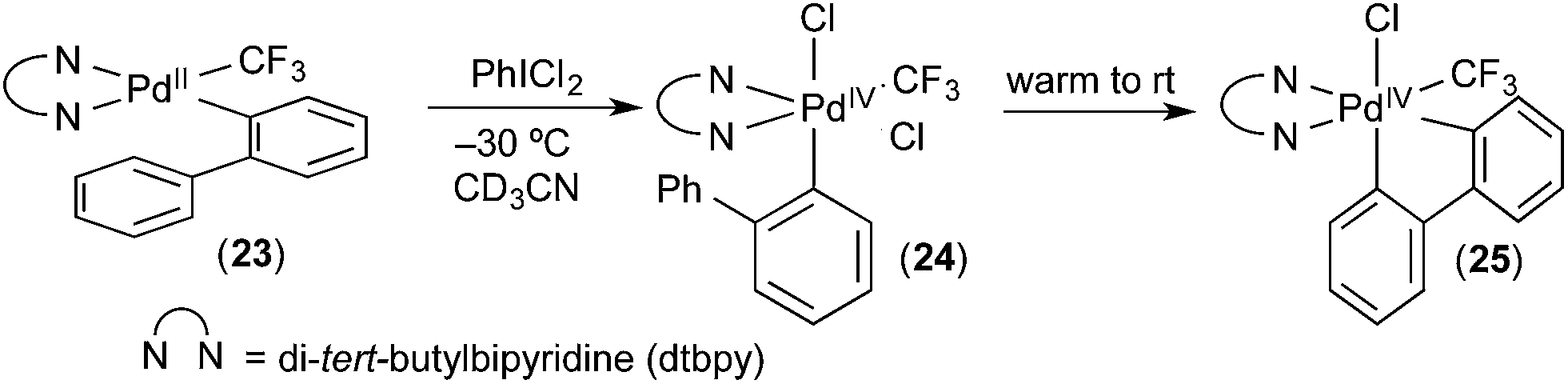
Carbon–hydrogen (C–H) bond activation at Pd IV : a Frontier in C–H functionalization catalysis - Chemical Science (RSC Publishing) DOI:10.1039/C4SC02591A

The mechanism of palladium(II)-mediated C–H cleavage with mono-N-protected amino acid (MPAA) ligands: origins of rate acceleration in: Pure and Applied Chemistry Volume 88 Issue 1-2 (2016)

Figure 1 from The mechanism of a ligand-promoted C(sp3)-H activation and arylation reaction via palladium catalysis: theoretical demonstration of a Pd(II)/Pd(IV) redox manifold. | Semantic Scholar

Proposed mechanism for Pd-catalysed oxidative coupling of 2aryl pyridines. | Download Scientific Diagram
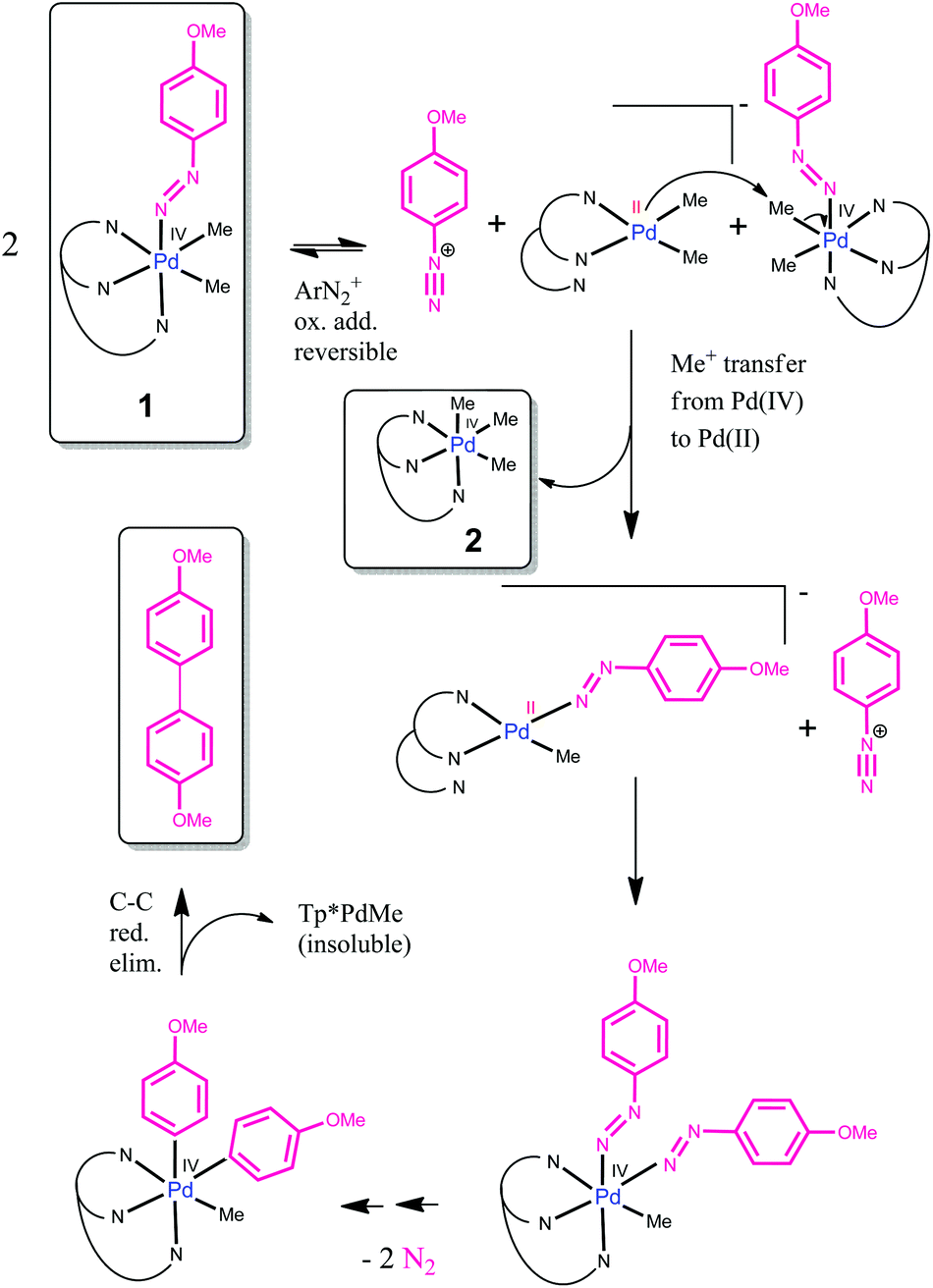
The first palladium( iv ) aryldiazenido complex: relevance for C–C coupling - Dalton Transactions (RSC Publishing) DOI:10.1039/C7DT00078B

Mechanism of the palladium(II/IV)-catalysed intermolecular C–H amination. | Download Scientific Diagram

Insight into the palladium-catalyzed oxidative arylation of benzofuran: heteropoly acid oxidants evoke a Pd(II)/Pd(IV) mechanism - ScienceDirect
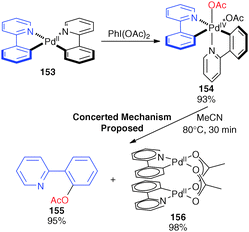
Experiment and computation: a combined approach to study the reactivity of palladium complexes in oxidation states 0 to iv - Chemical Society Reviews (RSC Publishing)
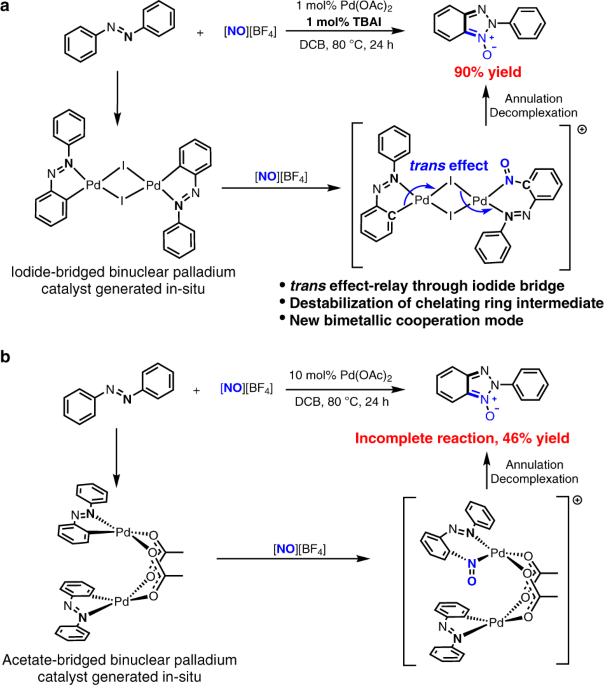
Iodide-enhanced palladium catalysis via formation of iodide-bridged binuclear palladium complex | Communications Chemistry
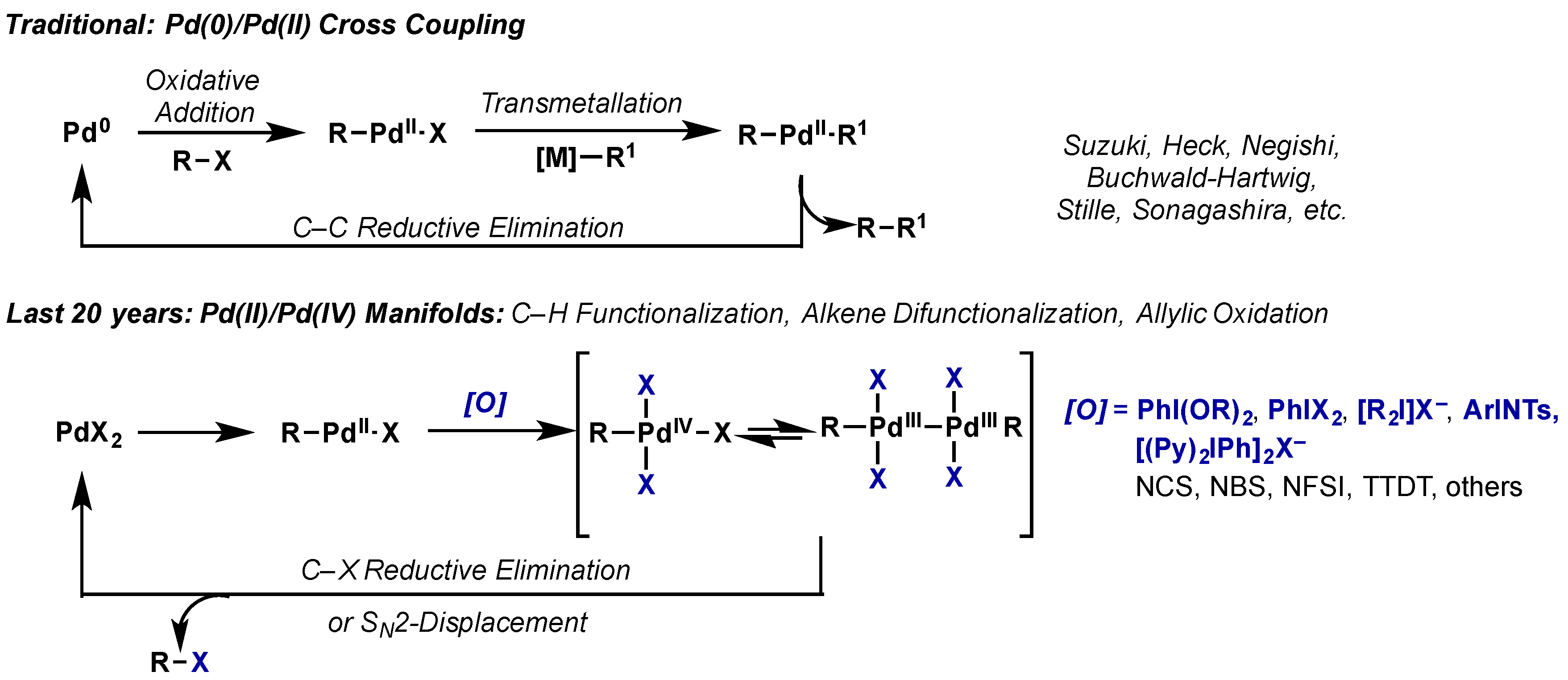
Molecules | Free Full-Text | Hypervalent Iodine Reagents in High Valent Transition Metal Chemistry | HTML
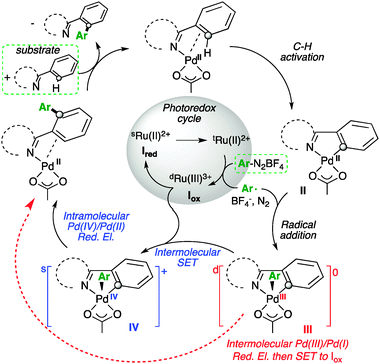
Radical Pd(iii)/Pd(i) reductive elimination in palladium sequences - Chemical Communications (RSC Publishing)

Figure 3 from The mechanism of a ligand-promoted C(sp3)-H activation and arylation reaction via palladium catalysis: theoretical demonstration of a Pd(II)/Pd(IV) redox manifold. | Semantic Scholar

Ligand‐Assisted Palladium(II)/(IV) Oxidation for sp3 CH Fluorination - Sun - 2016 - Advanced Synthesis & Catalysis - Wiley Online Library

Figure 7 from The mechanism of a ligand-promoted C(sp3)-H activation and arylation reaction via palladium catalysis: theoretical demonstration of a Pd(II)/Pd(IV) redox manifold. | Semantic Scholar

Scheme 1: Mechanism of palladium (II)-catalyzed oxidations of leucine... | Download Scientific Diagram

Report: Toward Greater Understanding and Expanded Utility of the Palladium-Catalyzed Activation of Carbon-Carbon Single Bonds (56th Annual Report on Research Under Sponsorship of The American Chemical Society Petroleum Research Fund)
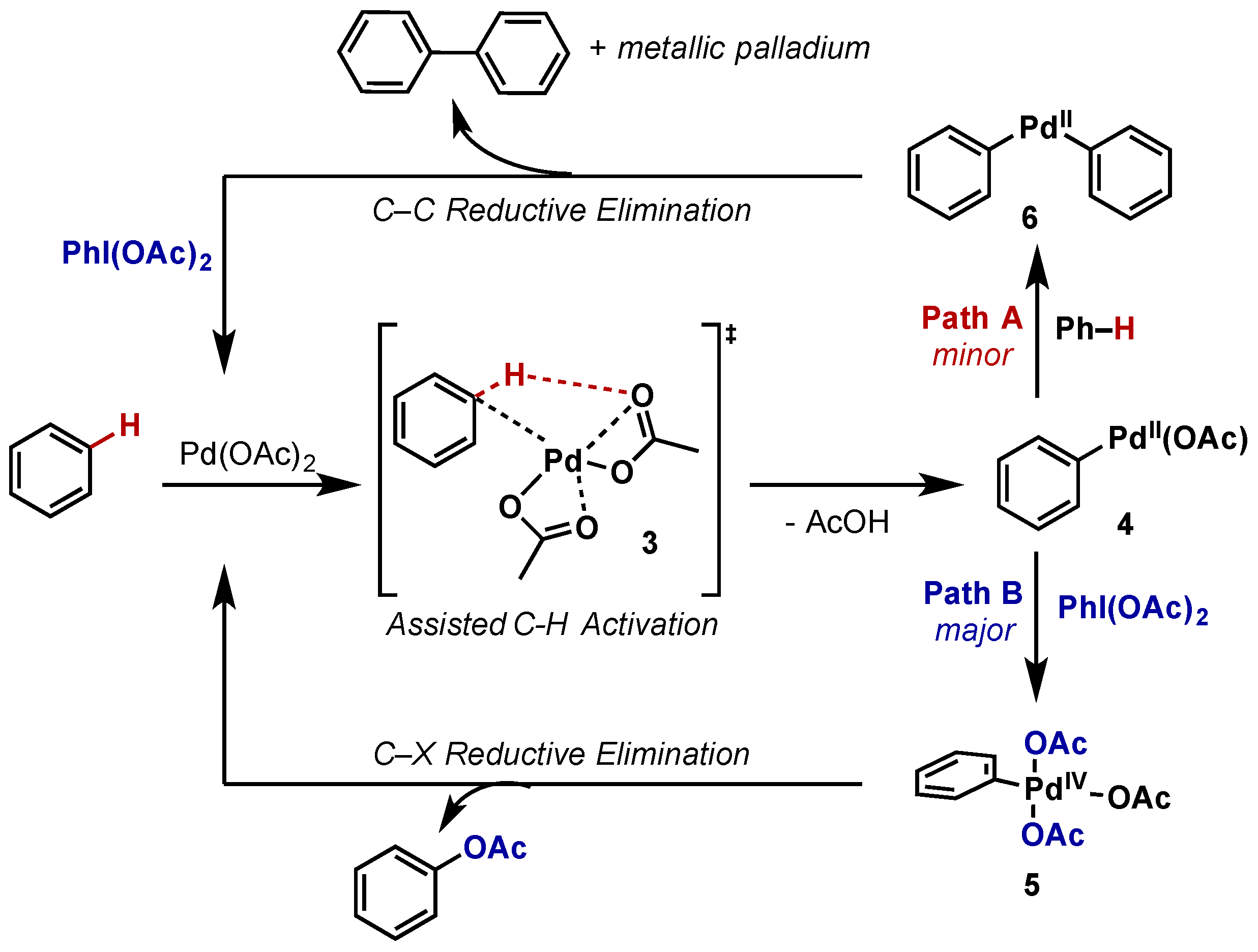
Molecules | Free Full-Text | Hypervalent Iodine Reagents in High Valent Transition Metal Chemistry | HTML